Healthy Feed Development & Conducting Animal-based Clinical Trials
Introduction
Feed Formulation Technology is becoming more significant due to numerous worldwide opportunities, difficulties, and threats ahead. Due to population expansion, rising incomes, and urbanization, there will be a 70% increase in the global demand for animal feed development in 2050 compared to 2000. For feeding 9 billion by 2050, the global demand for animal feed will reach 1500 megatons, with Asia and Africa experiencing the most increases. This drastic change coupled with increased awareness of zoonotic diseases, Covid-19 pandemic, increase in antibiotic resistance and extreme weather conditions has put tremendous pressure on animal feed industry to bring out sustainable outcomes. (1)
On the other side of the spectrum, clinical trials are essential for evaluation and documentation of the safety and efficacy of the any newly developed food or feed product. Before any human research is permitted, U.S. federal rules mandate that non-human animal research be carried out to demonstrate the safety and effectiveness of experimental treatments.
It is crucial to emphasize that animals constitute only one aspect of the broader process of biomedical research, and that 95% of all animals required for biomedical research in the United States are rodents—rats and mice specifically bred for laboratory use. Three key reasons for widespread animal usage in biomedical research are:

- Animals and humans are closely matched in biological spectrum. DNA of mice has a match of 98% with humans
- Animals and humans share similar type of health diseases such as heart diseases, cancer, diabetes etc.
- With shorter life span, animal models enable extensive study across generations and interaction within a living biological entity.
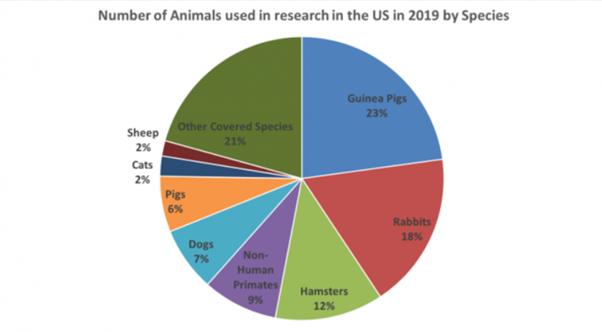
Source: Speaking of Research, 2021
Merits of Feed Formulation Technology:
Benefits of novel feed ingredient and formulation technology are:
- Generating a homogenous blend of diet constituents
- Reduce negative outcomes of anti-nutritional elements in ingredients
- Improve safety profile
- Increase feed efficiency
- Increase intake of feed with minimal spoilage
- Strike balance between feed competence and animal well-being
Hence, the feed industry will prioritize meeting user demands for security and resilience by boosting productivity and competence through new developments in feed additives and animal nutrition supplements, as well as advanced processing technology.( 2 )
Why Animal Model is Necessary?
The characterization of disease pathophysiology and related mechanisms of injury, the identification of drug targets, and the assessment of novel therapeutic agents for toxicity/safety, pharmacokinetics, pharmacodynamics, and efficacy have all benefited from the use of animal models in drug discovery and development. Animal models are typically used in drug discovery to establish and furnish proof for non-clinical “proof-of-concept” about the target of interest, safety, and effectiveness of certain pharmacological compounds.
Determining safety and efficacy characteristics is a crucial step not only for later regulatory approval for marketing and human use, but also for the early stages of drug research and development. Furthermore, creating clinical forecasts of the anticipated outcomes in people depends critically on the use of animal models.
For the discovery of new drugs, it is crucial to use animals with hereditary defects or induced diseases in addition to healthy ones. In summary, it is impossible to overestimate the role that these fundamental laboratory animals have played—and will continue to play—in helping us understand a wide range of basic and fundamental biological processes, such as the development of monoclonal antibodies, the screening of novel antimicrobials, the principles of immune tolerance, etc.
Challenges and Cure
Striving to prevent the infliction of unnecessary pain or suffering on animals, the Prevention of Cruelty to Animals Act (PCA Act-1960) in India mandates that the Committee for Control and Supervision of Experiments on Animals (CPCSEA) “take all such measures as may be necessary to ensure that animals are not subjected to unnecessary pain or suffering before, during, or after the performance of experiments on them.”
An estimate from 2010 states that up to US$240 billion has been invested globally in biomedical research, with fundamental research benefiting the most. When it comes to applied research, many of the greatest research concepts that promised translational effects to have fallen short. Because of the bottleneck effect this has produced, we are now questioning the usefulness of basic research in creating protocols for the prevention and treatment of disease. It takes about 15 years from approval to market launch and the cost of development is nearly $1.3 billion.
Few ways to prevent exploitation of animals during trial studies are:
- Provide accurate justification of animal model use
- Perform extensive and systematic literature review (as shown in the figure)
- Building study design based on internal and external validation
- Take ethical, moral values into consideration
- Incorporation of humane end point
- Accurate understanding of the test animal in terms of physiological, pathological, and behavioral patterns
- Suitable housing environment
- Employing power calculations (2 )
Conclusion
Feed technology must assess every facet of existing methods to enhance feed and ingredient processing and preservation technologies to meet the demand for new feed parts. When it comes to animal clinical trials, it is important to employ refined study designs, statistically large sample size, ethically suitable protocols for good translational outcomes, and reproducibility.
Guires FRL CDMO is a one-stop solution to all your feed development and animal clinical trial needs. Be it creating a novel nutritive feed from scratch or reverse engineering a popular feed in market we let you use our years of experience in animal product development to bring life to your innovative idea and meet your expectations. Apart from formulation and developing, we also support you with extensive clinical trials and regulatory support and extend a helping hand till your product is successfully launched in the market.
References:
- Singh, V. P., Pratap, K., Sinha, J., Desiraju, K., Bahal, D., & Kukreti, R. (2016). Critical evaluation of challenges and future use of animals in experimentation for biomedical research. International Journal of Immunopathology and Pharmacology, 29(4), 551-561.
- Van der Poel, A. F. B., Abdollahi, M. R., Cheng, H., Colovic, R., Den Hartog, L. A., Miladinovic, D., … & Hendriks, W. H. (2020). Future directions of animal feed technology research to meet the challenges of a changing world. Animal Feed Science and Technology, 270, 114692.
- Den Hartog, L. A., & Sijtsma, R. (2013). Challenges and opportunities in animal feed and nutrition.