
Clinical trials for nutraceutical and checklist for outsourcing
Introduction:
Nutraceuticals are formulated nutrients that, when added to a supplement diet, aid in the prevention and treatment of certain diseases. However, it is confusing due to several other terminologies like ‘supplements’ and ‘functional foods.’ One major differentiation is nutraceuticals can aid in disease prevention or immunity augmentation backed by strong scientific evidence, as shown in Figure 1. This makes a strong case for nutraceutical clinical research through extensive clinical trials.
One of the most crucial and expensive phases of the drug development cycle is clinical development. Clinical trials are thought to cost over $800 million for each authorized medication. Hence, there is immense pressure on biotech and pharmaceutical firms to reduce clinical development costs.
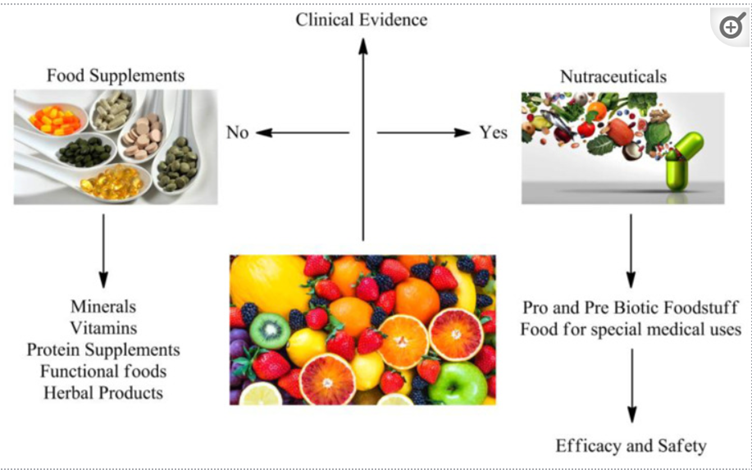
Source: A comprehensive review on nutraceuticals: therapy support and formulation challenges
Unique Challenges in Nutraceuticals
Let’s look at a pharmaceutical company that generally sponsors drug-based clinical trials. It has all the necessary technical proficiency for generating numerous regulatory documents that are mandatory to secure approval from federal governing bodies like Health Canada, the Therapeutic Goods Administration (Australia), and the US Food and Drug Administration. Employees at large pharmaceutical companies will be skilled in creating ethics proposal documents and Clinical Trial Application (CTA) forms for their trials. One cannot find the same level of expertise when it comes to nutraceutical and functional food firms. Creating a CTA for functional food might be a daunting task for a principal investigator working alone or even in a hospital-based research team unless they have easy access to this kind of knowledge. Several other unique problems when it comes to nutraceutical clinical trials are as follows:

- Identification of an apt placebo: In crossover trials, the longer duration with functional foods could lead to increased budget, reduced compliance, loss of subjects, and advancement of the disease
- Delivery system: Functional food necessitates frequent trips to the location where it is obtained or regular meal delivery to the trial participants.
- Quality and Sensory values: The microbiological and organoleptic quality of the functional food should be maintained
- Restricting Access: Functional foods are available in every supermarket, which gives subjects easy access.
- Statistical Analysis: Statisticians are often unavailable or come in at a later part in the planning of the trial, which is not ideal
- Data Management: Inaccurate data due to shortened clinical trials and distrust due to lack of peer-reviewed results in nutraceutical research
One grand solution to this problem at hand would be sourcing out clinical trials of your nutraceuticals to renowned contract research organizations (CRO) either locally or internationally. 1
CROs to your Rescue
Clinical CROs are companies that offer services focused on managing and carrying out clinical development. Their services include developing protocols, registering with regulators, identifying study locations and principal investigators (PIs, the doctors in charge of the trial), reviewing clinical samples, and statistical analysis, among many other tasks.
The major functions of a CRO are as follows:
- Offering instant access to clinical development faculty
- Providing financial flexibility through using infrastructure and other material resources
- Allowing businesses to center their attention on other core issues
On the contrary, the core competence of a clinical CRO is clinical trial management. Several different clinical trials managed by a CRO share the overhead, infrastructure, human resource network, and training costs. CROs have been capable to completing clinical trials 25-30% faster than in-house and still maintaining a similar quality output.
According to estimates, around 18.9% cutback in drug development time would save $100 million cost and a 41.3% reduction would save $200 million. Therefore, outsourcing to CROs can dramatically lower the cost of any drug/functional food development. 3
Checklist for Selecting CRO:
Before closing in on the right CRO for outsourcing your nutraceutical clinical trials, the following key facts in CRO selection are necessary to ensure a smooth and effective relationship.
- Collaborative chemistry: It is important to ensure that the CRO shares the same morals, business ethics, and working methodologies as your firm.
- Experience and Portfolio: When assessing CROs, it’s critical to evaluate the company’s experience, particularly in your therapeutic area. Examine their past performance to see if they have regularly met their clients’ needs.
- Turnaround Time: Look deeply into the CRO’s track record of providing real-time responsiveness, efficient execution of protocols, and strict adherence to deadlines
- Problem-solvers: To enable prompt adaptation to unforeseen events that frequently arise during a trial, make sure that all members of the fully integrated team—both sponsor and CRO—have real-time access to data. It is seen that 47% of companies consider this as a vital factor in choosing a CRO.
- Expertise and Infrastructure: Due diligence should be given to whether the CRO has sufficient facilities and skilled or high expertise in the field of interest to meet your project requirements. An appropriate CRO infrastructure is necessary to support your project requirements. 2
Conclusion:
Even though they are pros in choosing global contract R&D clinical trials and services, companies do lose a great deal of control over the administration of their activities and their potentially vulnerable intellectual property (IP), even while they still retain overall control over their products. As a result, outsourcing clinical trial management calls for a high level of transparency and effective communication. Enforcing IP protection requires a sufficient infrastructure.
This is where Guires FRL CDMO comes as a great blessing. We have a robust infrastructure, skilled experts, proven experience in carrying out successful clinical trials in functional foods, and a crystal clear line of communication and documentation processes to reassure the trust and confidence of our clients.
References:
- Brown, L., Caligiuri, S. P., Brown, D., & Pierce, G. N. (2018). Clinical trials using functional foods provide unique challenges. Journal of Functional Foods, 45, 233-238
- Glass, Harold E; Beaudry, Daniel P. Key Factors in CRO Selection, Applied Clinical Trials; Monmouth Junction Vol. 17, Iss. 4,(Apr 2008): 52,54,56,58,60.
- Yang, H. (2008). Strategic analysis of clinical trial outsourcing to China.





