Why is India a Popular Destination for Clinical Trials?
Introduction
There is a greater emphasis on lowering the cost of clinical development due to the increasing pressure on the global pharmaceutical sector to manage R&D (research and development) costs. New drugs launches are also being negatively impacted by the delayed development issue, which results in a loss of incremental revenue. Clinical trials are seeing exponential growth in several new areas as well.
Nowadays, it’s getting harder and harder for pharmaceutical corporations to get enough volunteers to test the medications that come out of their labs. For an experimental medicine to be approved for marketing by the Food and medicine Administration, an average of about 4000 patients must be involved. However, in the US, less than 5% of patients are open to taking part in clinical trials. In the United States, 86% of clinical studies do not enrol the necessary number of participants and are delayed by an average of 366 days. Revenue is lost every day by one million dollars due to product delays in reaching the market.
Due to the dual challenges of cutting expenses and speeding up clinical development, big pharmaceutical companies are now searching for other locations to source people for their international trials. Research along these lines prompts the pharmaceutical sector to show interest in nations such as Asia, Eastern Europe, and Latin America. India is a standout Asian nation because of its enormous population of treatment-naïve patients, English-speaking doctors, and a strong pharmaceutical industry that has dominated the global market thanks to low-cost generics.1
How does India solve the Problem?
After signing the TRIPS agreement, the 1970s Patent Act had to be modified to give Indian businesses the same protection for their inventions as businesses worldwide. This encouraged Indian businesses to refocus their efforts from developing generic pharmaceuticals to manufacturing novel chemical entities. Prior to 2005, there weren’t many pharmaceutical MNC trials carried out in India. The International Conference on Harmonisation (ICH) standards were added to Schedule Y of the Drugs and Cosmetics Act of India by the Indian government in January 2005. A number of regulatory obstacles to conducting clinical trials in India were eliminated by these modifications. Some of the significant changes are highlighted in the diagram below: 2
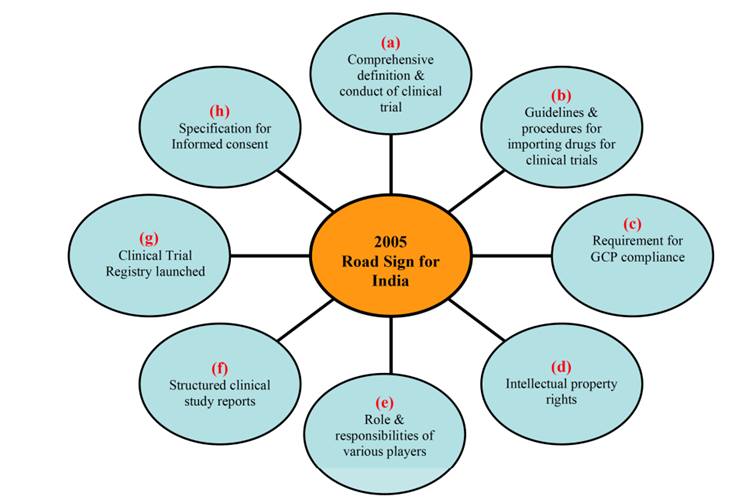
Source: Realizing the Promise: India’s Strategic Shift from Outsourcing to Innovation
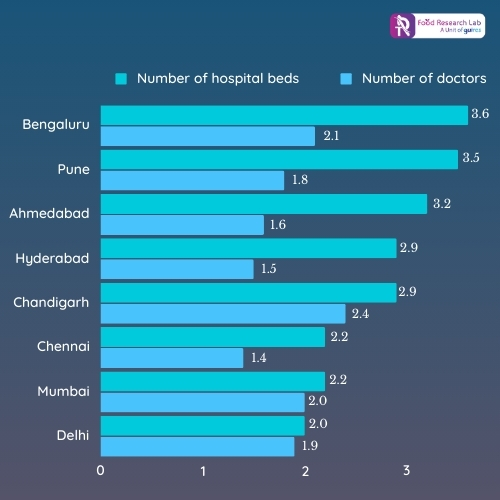
Looking at the graph 1, it is evident that the number of clinical trials occurring in India has risen from 4% in the last decade to over 10% since 2013. One of the key factors in the dramatic change is excellent infrastructure and skilled doctors in India as depicted in graph 2. The other important factors are as follows:
- Conducive regulatory environment
- Cutting-edge research and technology programs
- Diverse subject population
- Cost-effective pricing
- Hassle-free recruitment
Graph 1:
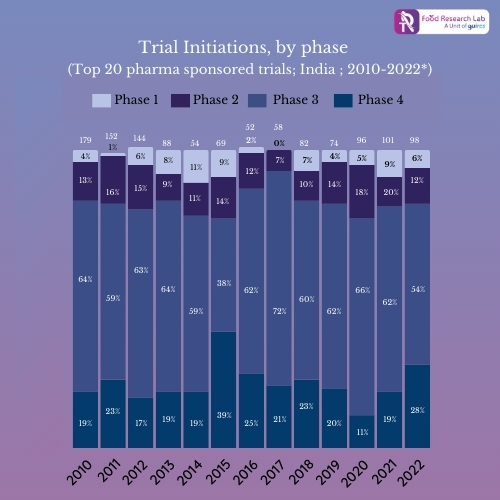 Graph 2:
Graph 2:
The Emergence of CROs
Contract research organization (CRO) took a ‘go global’ approach with experience of working in different national and cultural boundaries. They changed the landscape of clinical trials in India by introducing revolutionary research methodologies, new transferrable skills, and helped increase the clinical trial climate in India. However, more research is required to understand how established practices are adapted to these new settings and how they change inside these new locales. The outsourcing of clinical trials by multinational pharmaceutical corporations seeking untapped markets and treatment-naïve patients is transforming the current pharmaceutical industry, harmonising it to more closely resemble global or “Big Pharma.” Big-pharmaceuticalization entails the entry of CROs into India that carry out research for both domestic and foreign pharmaceutical corporations. As independent actors, CROs collaborate with sponsors, physicians at patient recruitment trial sites, and occasionally biotechnology businesses conducting basic research. They reaffirm how Randomised Control Trials (RCTs) take centre stage in a specific type of knowledge generation and start to supplant earlier generic medication production systems.
Challenges Faced in India
Some of the obstacles faced in conductance of clinical trial in India are as follows:
- Regulatory bottlenecks exist, and approval processes are still taking a very long period. India’s regulatory bodies are terribly slow in comparison to those in Canada and the UK, where clearance processes take 30 days. This is a result of insufficient financing and regulatory staff training.
- One of the most significant challenges the industry is having to draw in a lot of foreign clinical trials to India is the lack of hospital locations that meet ICH-GCP standards.
- A growing number of CROs are emerging without the necessary quality accreditation. The government ought to halt the operations of CROs that are not accredited.
- Education is a major obstacle to patient compliance. Since a large number of the patients in the trial scenario are from rural and semi-urban areas, it is important to make sure they are aware of compliance difficulties and have a good education. 3
Conclusion:
Despite a few challenges that need to be ironed out, India is well-positioned to provide the international pharmaceutical industry with high-quality, reasonably priced contract services to aid in the discovery of new drugs. Added to this, the growth of CROs in India is becoming evident as they play a major role in bringing in technological advancements, cost-effectiveness, fastening the approval process, and so on.
We at Guires FRL CDMO possess a highly skilled and expert team of scientists at your disposal, capable of overseeing every brick of clinical trials from planning to reporting without compromising on quality standards laid down by WHO guidelines, GLP standards, and ICH guidelines. We guarantee high standards of safety, quality, and ethical practice with a lightning-fast turnaround timeline.
References:
- Sunil, S., & Sameer, P. (2013). Realizing the Promise: India’s Strategic Shift from Outsourcing to Innovation. The Open Clinical Trials Journal, 3(1).
- Sariola, S., Ravindran, D., Kumar, A., & Jeffery, R. (2015). Big-pharmaceuticalisation: Clinical trials and contract research organizations in India. Social Science & Medicine, 131, 239-246.
- Garg, T., Singh, O., & Arora, S. (2011). Opportunities and growth of conducting clinical trials in India. Int J Pharm Sci Rev Res, 8(1), 152-160.