How Guires FRL CDMO Meets Nutraceutical Stability Testing in Veterinary
Introduction
Much has been written since the late 1970s regarding the potential use of foods as medicine to support longevity and good health. Unfortunately, the Food, Drug and Cosmetic (FD&C) Act would classify such goods with a disease-like claim as medications, therefore food makers were prohibited from taking that approach. This was altered by the 1992 Nutritional Labelling and Education Act (NLEA), which permitted specific health claims to be made on foods. Only Few food products managed to have fulfilled the strict criteria needed to qualify for a health claim.
However, in the veterinary sector, the growth of nutraceuticals has been manifolded. Over the past 30 years, the usage of nutraceuticals for veterinary purposes has grown significantly, making them a billion-dollar industry. This pattern indicates a change in the perspectives of veterinarians, manufacturers, and animal owners. Conventional pet food and dietary supplements provide a variety of nutritional options for disease management and health promotion, providing an alternative to relying exclusively on pharmaceutical or medical goods for disease prevention and treatment. Thus, need for new nutraceutical formulations and product development have drastically grown and ensuring its stability is vital 2
Stability Testing – Why is it Needed?
In pharmaceutical industry, stability of active ingredients and manufacturing is strictly moderated with several regulations. This is quite absent in case of nutraceutical sector. From a regulatory perspective, expiration dates are not necessary on labels for nutraceuticals in the United States. This has given rise to legitimate worries regarding the effectiveness of nutraceuticals and their stability over time when utilised in both veterinary and human settings. Major reasons for shelf-life testing of nutraceuticals are as follows:
- Concern for the health of the animal afflicted with the medical condition for which the product is intended
- Ensure that the product will be fit for use in terms of all functionally relevant aspects throughout the duration that it is sold, protecting the manufacturer’s reputation in the process.
To ensure that a nutraceutical formulation in veterinary research combines quality, efficacy, and safety, stability testing is a must. It is a sophisticated series of processes requiring significant money, time, and scientific skill.
The ability of a substance or product in a particular container-closure system to stay within specified parameters to maintain its identity, strength, quality, and purity throughout the retest or expiration dating periods is known as stability/shelf life for drugs or nutraceuticals. Thus, stability testing assesses how external influences affect the quality of a manufactured product or nutraceutical substance, which is used to forecast its shelf life, choose the best storage settings, and recommend labelling guidelines.
Roughly 90% of veterinarians in the USA provide innovative ingredients, like nutraceuticals, for sale. Almost thirty percent of pet owners have either used or are thinking about using innovative components in their animals, like herbs and botanicals and nutraceuticals. Although product expiration dates are frequently searched for by consumers, nutritional supplements are not currently required by US federal rules to include an expiration date. It is the goal of manufacturers to achieve a minimum of two years of stability for their nutraceutical goods, which means that if a retest is conducted two years after manufacturing, the substances indicated as “active” on the label will still be able to match the claim made on the label.
The best a consumer can do is look at the label to find out the manufacturing date and hope that, with proper storage, the product will remain stable for at least two years. It is important for manufacturers, veterinarians, and consumers to understand that not all nutraceuticals live up to the label.
Factors involved
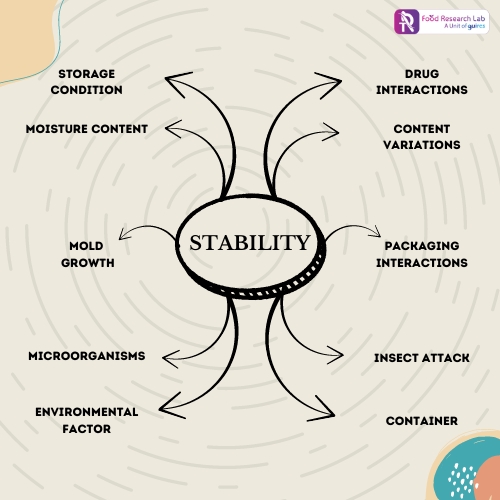
Numerous factors can impact the stability and concentration of nutraceuticals in both their unprocessed and processed forms. These include the state of the environment, unstable chemicals, interactions between matrices, packaging, and other intangible problems. The stability of a nutraceutical product can be affected by changes in its appearance, consistency, homogeneity of content, clarity (solution), moisture content, particle size and shape, pH, and package integrity. Such physical alterations could result from shearing, freezing, thawing, impact, vibration, abrasion, and other temperature variations. Nutraceutical products may undergo chemical reactions such as solvolysis, oxidation, reduction, racemization, etc. that result in the formation of degradation products, a decrease in the potency of the active ingredient, and a loss of excipient activity such as antioxidant and antimicrobial preservative activity as depicted in below figure. 1
Conclusion
Many of the tests used in a stability evaluation will be specific to the product, but they will typically fall into one of four categories:
- sensory/organoleptic evaluation (colour, odour, and taste)
- chemical analysis (assays for active component levels)
- physical analysis (taste, hardness, and tablet assessments)
- microbiological examination (based on assessments of microbiological risks).
The nature and characteristics of the product and the ingredients, the suggested label claims, the packaging and expected storage conditions, and the shelf life necessary for commercial viability are just a few of the variables that will determine which tests are chosen for any given ingredient or product.
Looking for stability testing of your formulation or innovating on nutraceuticals? Guires FRL CDMO is simply an expert at this. Possessing a team of high expertise in variety of testing methodologies like genomic/gene expression studies, in vitro bioassays, and human clinical testing, we provide consistent and innovative solutions for all your requirements related to nutraceutical formulations and its stability.
References:
- DuBourdieu, D. (2019). Veterinary Nutraceuticals Stability Testing. Nutraceuticals in Veterinary Medicine, 765-774.
- LeDoux, M. A., Appelhans, K. R., Braun, L. A., Dziedziczak, D., Jennings, S., Liu, L., … & Griffiths, J. C. (2015). A quality dietary supplement: before you start and after it’s marketed—a conference report. European journal of nutrition, 54(Suppl 1), 1-8.