Clinical Trials for Cosmeceutical and Checklist for Outsourcing
Introduction
Cosmetics are a fast-expanding sector, and there’s no denying that cosmeceutical products play a major role in modern day care. With record-breaking sales since the early 2000s, the cosmeceutical sector has grown to be a sizable consumer-driven market, with US$9.7 billion in sales in 2011 and US$12.3 billion in sales predicted by 2025. 1The origin of cosmeceutical can be traced back to about 30 years ago, coined by Albert Kligman. They comprise a list of products that possess biological ingredients with drug like advantages. It may not be permissible to use the terms “evidence-based” and “cosmeceutical” together. Therefore, their active ingredients should have a solid scientific rationale based on human clinical trials findings. There are two common types of cosmeceuticals are available in the market:
- Drug form
- Over the Counter
The growing desire to appear younger and healthier has led to enormous commercial opportunities and has taken on global importance. Social media and the internet’s influence have increased public knowledge of the health advantages of natural products made from plants and other natural resources as well as the concerns connected to the use of various chemicals in cosmetics. The cosmetics business is currently focusing more on natural goods as a result. This is where Guires FRL CDMO can be great problem-solvers for you, we are pioneers in cosmeceutical product formulation and development from plants and botanical extracts.
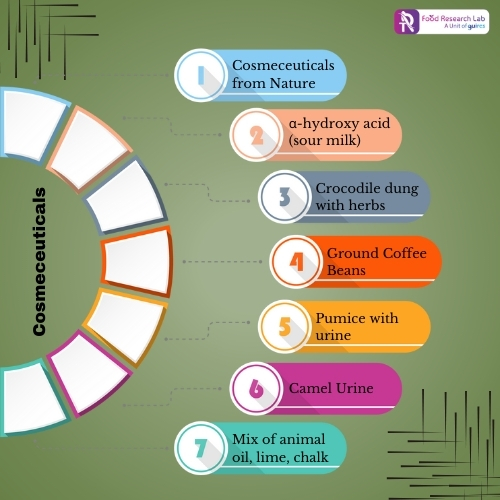
Below image shows list of natural interventions used in ancient history to improve skin health.
Less Evidence Base
Since the cosmetic industry is not bound to federal regulations under FDA rules and there is no necessity to exhibit premarketing safety or efficacy. Hence millions of extracts derived from botanical and synthetic sources can be fused into cosmetics.
It is therefore appropriate to determine the benefits and downsides of treatments that are readily accessible commercially and that dermatologists prescribe, considering the abundance of treatments with questionable efficacy. Finding frequently used products that make claims about their effectiveness and safety is also crucial. Consumers are searching for a magical solution that is affordable, easy to use, and effective more than ever. Dermatologists frequently visit consumers who inquire about new products that promise to remove wrinkles in only one week or if certain products are useful. Understanding the composition and functionality of human skin is necessary to address these questions with a clinically and scientifically accurate technology. For proper evaluation of a novel cosmeceutical product, claiming to have a beneficial effect, it is critical to ask the following four questions: 3
- Does the active ingredient have good penetration to stratum cornea and find its way to the intended target cell at adequate concentration and understand its action mechanism?
- Can we understand the biochemical mode of action on the target cell?
- Is the beneficiary supported by strong evidence from peer-reviewed, placebo-controlled, double-blinded, and statistically substantial clinical trials?
- Does it have a low risk profile?
This iterates the need for extensive clinical research should be followed cosmeceutical product development that is to be placed on the market. The development of cosmeceuticals, particularly composite active ingredients, should be grounded in their well-defined sources, structures, and interactions with the skin; above all, it should be based on their safety and effectiveness when applied to the specific skin components.
Hence for cosmetic products to carry a “clinically tested” claim, it is important to undergo rigorous clinical trial conforming to the standards set by Food and Drug Administration (FDA).
In the view of rising global challenges from Brexit to Russia-Ukraine war, volatile international relations, occurrence of pandemics and so on, has caused several delays and issues in research and drug development cycle. Outsourcing of clinical trials is seen as a blessing in disguise by many companies since it allows them to reduce costs, largely decreases the time to reach the market, and has simplified the patient recruitment processes.
Checklists to be Followed:
Even though there are many pros in outsourcing clinical trials, it does come with a lot of risks such as transparency and confidentiality issues, unseen delays among several others, Hence selecting the right outsourcing contract research organisation (CRO) is crucial. Let us look at a few important points to be followed while outsourcing a cosmeceutical clinical trial as per FDA is as follows:
- Overview of the Company: Check historical background, financial stability, ability of the CRO to manage projects under unforeseen circumstances.
- Quality Accreditations for CROs: All current and past certifications and licenses like Good clinical practice (GCP), Good Laboratory practice (GLP), National Accreditation Board for Testing and Calibration Laboratories (NABL), etc.
- Investigational site and investigator relationships: CRO should have a larger database with excellent bond with investigators is essential for easy site selections and study completion
- Infrastructure and Technology: Ensure the CRO has the latest technology and research tools to carry out the clinical trial within the stipulated period
Conclusion:
The concept is by no means novel or false to suggest that substances originating from nature can be curative or even reverse illness processes. Limited cosmeceuticals comprise active ingredients with evidence-based corroboration of efficacy. But with more consumers starting to become more intelligent about product and marketing claims, substantial evidence is being required. Hence there is an urgent need for effective and efficient cosmeceutical clinical research management.
This is where Guires FRL CDMO comes into the picture. We have a team of experienced and talent professionals who develop trials which aim to prove the safety of product, efficacy, substantiate the specific advertising claims and help your business standout from the competition.
With a variety of study options, including consumer perception surveys, safety and efficacy studies, randomized-controlled trials, we at Guires FRL CDMO help in streamlining and easing the process of evaluating skincare products. Speak with us right now to experience the impact of scientific validation.
References:
- Emily C. Milam;Evan A. Rieder; (2021). An Approach to Cosmeceuticals. Essential Psychiatry for the Aesthetic Practitioner, (), –. doi: 10.1002/9781119680116.ch4
- Desai, S., & Patel, N. (2021). Checklist to select contract research organization for early phase bioavailability/bioequivalence clinical studies in healthy adult human volunteers. Research Journal of Pharmacology and Pharmacodynamics, 13(4), 131-142.
- Monteiro, Erica de O; Baumann, Leslie S (2006). The science of cosmeceuticals. Expert Review of Dermatology, 1(3), 379–389. doi:10.1586/17469872.1.3.379
- Liu, J. K. (2022). Natural products in cosmetics. Natural Products and Bioprospecting, 12(1), 40.