What Makes a Claim Acceptable Under EU Law?
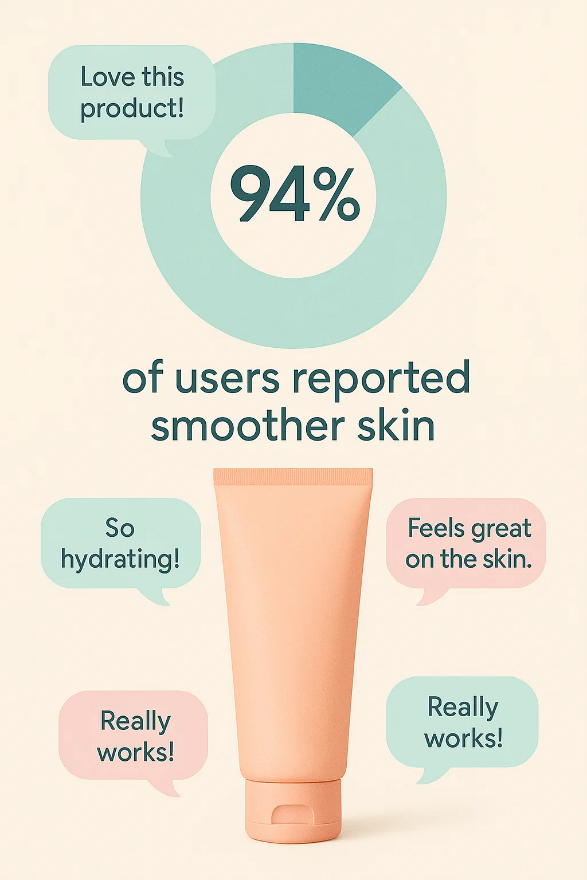
Under Article 20 of the CPR, claims made about cosmetic products—whether on packaging, in advertising, or online—must not be misleading and must be substantiated by appropriate evidence.
To further guide the industry, the European Commission introduced the Common Criteria for Cosmetic Claims, structured around six principles: