Young Adults
Our goal is to make your dream concept a commercial product, integrating our strong knowledge of ingredients and techniques to help you make the right decisions
Our goal is to make your dream concept a commercial product, integrating our strong knowledge of ingredients and techniques to help you make the right decisions
Our procurement team from Guires FRL hunt for the latest innovations to identify best practices globally to develop geriatric food products.
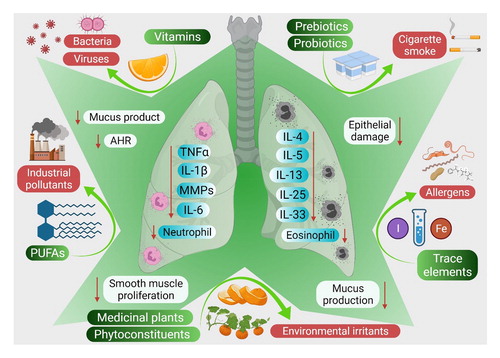
Persistent respiratory tract inflammation contributes to various chronic diseases, including asthma, chronic obstructive pulmonary disease and pulmonary fibrosis. Various nutraceuticals, including prebiotics, probiotics, phytochemicals and vitamins, have gained attention because of their therapeutic potential in modulating pathological mechanisms underlying inflammatory respiratory diseases, thereby enhancing disease control and overall health outcome. Our formulation experts develop an evidence-based product considering high-quality studies. There is evidence that several probiotic strains (LGG, Lactobacillus rhamnosus; Lactobacillus reuteri DSM 17938 250–500, Lactobacillus casei DN-114 001, Lactobacillus casei Shirota) and prebiotic oligofructose evidence to reduce the incidence and duration of respiratory tract infection. Similarly, both EPA and DHA have been found to reduce the symptoms and occurrences of bronchial asthma and COPD. Studies have shown that red and green algae as a potential source of EPA and DHA, other sources such as modified Camelina Sativa L, nitrate in plant-based diet such as beetroot juice enhances physical performance in COPD patients. Similarly, Vitamin C, E, D, polyunsaturated fatty acids (rise in n-6 and decrease in n-3), saffron, Fish, Green tea, Flavonoids, Spirulina, magnesium, curcumin, Quercetin, Nigella Sativa, Lipoic Acid, Glycine, Selenium, Zinc, Citrulline, Folate, NAC, Biotin, Taurine, and Magnesium either support inhibition of NADPH Oxidase complexes or support Glutathione Synthesis and expression of antioxidant enzymes in the body.
Our experts also formulate products targeting asthma from botanicals and herb extract such as Ganoderma lucidum (fungal), Essential oil of Eucalyptus globulus leaves, P. rotundum var. subintegrum extract, Achyranthes aspera L, Allium Cepa L. and quercetin, Andropogon muricatus, , Anoectochilus formosanus Hayata, Aster tataricus L. f. Baliospermum montanum Müll. Arg. (Euphorbiaceae)Sophora flavescens, and Glycyrrhiza uralensis (Fabaceae species)), Gamma linolenic acid (GLA; borage oil) , Licorice, and Boswellia serrata. These compounds have been promoted for antiasthma activity due to their anti-inflammatory and bronchodilator properties. We can develop supplements or products using a single nutrient or a combination of several nutrients in the form of ready-to-mix powders or as a meal kit. Our team also offer help in looking at the pharmaceutical activity, including pharmacokinetic study regarding their efficacy, safety and the required dosage of the products from animal and microorganisms sources to induce in vivo antiasthma activity.
The prevalence of arthritis is increasing among many types of arthritis; osteoarthritis (OA) and rheumatoid arthritis have been more prevalent. Various natural food products have their own potential to regulate or induce inflammation. For instance, flavonoids and polyphenols from tea and pepper have been used to treat diseases. Presently, the use of nutraceuticals appears to be a good option to treat or manage OA because they are taken orally, well-tolerated, and safe.
Our food formulation scientists develop an evidence-based product formulation to meet the actual requirements of your needs. Evidence showed that Glucosamine sulfate, hyaluronic and Chondroitin Sulfate (GAIT Intervention trial, Clegg et al., 2005) are glycosaminoglycans (GAGs) evidenced to protect the joints. Similarly, Methylsulfonylmethane, olive oil, undenatured Type II collagen derived from Chicken Sternum Cartilage, cod liver oil, pomegranate extracts, sulphoraphane, conjugated linoleic acid, rose hip extract, Avocado/soy unsaponifiable (ASU), oleoresin from the Boswellia serrata tree, curcumin, Harpagophytum procumbens is a South African plant known as devil’s claw. , Kulattha (Macrotyloma uniflorum Lam. or Dolichos biflorus) , ginger, moringa, barley, and garlic.
Recently there are several products on the market, such as Logic Juice 4 joints, a juice-based product with added chondroitin and glucosamine, pure cod liver oil, joint care glucosamine with chondroitin plus fish oil omega 3. Our formulation expert can develop supplements or products using a single nutrient or a combination of several nutrients in the form of ready-to-mix powders or as a meal kit. Our team also offer help in looking at the pharmaceutical activity, including pharmacokinetic study regarding their efficacy, safety and the required dosage of the products from animal and microorganisms sources to induce in vivo anti-inflammatory activity.
Multiple Sclerosis is an immune-mediated, chronic, inflammatory, demyelinating neurodegenerative disease affecting the central nervous system. Nutraceuticals such as phenolic acids, vitamins, non-flavonoid polyphenols, flavonoid polyphenols, antioxidants (e.g., vitamin A, vitamin E, lipoic acid, and coenzyme Q10), melatonin, diterpenes, and organic sulfur compounds have all been shown to possess an anti-inflammatory and neuroprotective effect against multiple sclerosis. Beside, Echinacea, ginseng, CoQ10, ginkgo Biloba, DHEA, and garlic may decrease the effectiveness of the primary medication used to manage MS. In fact, the FDA has approved Omega-3 fatty acids and considered 1 to 3 grams daily safe intake. Herbals also have potential rejuvenating and immune-modulatory properties with negligible side effects. Our formulation team can develop products in the form of a diet kit or supplementation to reduce the risk and slow the progression of the disease. Our team also offer help in looking at the pharmaceutical activity, including pharmacokinetic study regarding their efficacy, safety and the required dosage of the products from animal and microorganisms sources to induce in vivo anti-inflammatory activity.
Gastric ulcers are triggered due to an imbalance between the quantity of acid secreted and the mucous protection barrier, leading to stomach or duodenum mucosa lining damage. Our formulation scientists developed an evidence-based product based on research. For instance, Liquorice dried peeled or unpeeled roots and stolons of Glycyrrhiza glabra Linn have evidence of anti-inflammatory and anti-ulcerative effects as it increases prostaglandin concentration in the digestive system promoting stomach mucus secretion. Similarly, C. dactylon’s , Probiotics have been shown to treat non-infectious through inhibition of pathogenic intestinal bacteria. In fact, these raw materials are found to prolong the neuron's lifespan in the stomach and have an antipepsin effect.
Our team also offer help in looking at the pharmaceutical activity, including pharmacokinetic study regarding their efficacy, safety and the required dosage of the products from animal and microorganisms sources to induce in vivo anti-inflammatory activity. Based on your requirement, we use a standardized methodology (e.g., aqueous extract, ethanolic extract, alcoholic extract, hydro-alcoholic extracts) to extract large amounts of biologically active compounds or phytochemical compounds.
The prevalence of chronic diseases such as diabetes, coronary heart disease, rheumatic heart disease, peripheral arterial disease, diabetes and cancer are high among men. This is due to inflammation-induced endothelial abnormalities and poor dietary habits along with unhealthy lifestyles. Phytochemicals, in general, are evidenced to improve human health as fresh plant products like fruits & vegetables, or at the other extreme as high value-added processed forms. We develop functional foods and beverages and nutraceuticals in the form of dietary supplements, herbals and nutrients. Our team has experience in formulating products using ketogenic diets, whole grain components, phytoestrogens, peptides, hydrolysates, dairy foods, polyphenols (phenolic acids, flavonoids, stilbenes, lignans and polymeric lignans), prebiotics, probiotics, spices, dietary fibre, postbiotics, and PUFA. Our formulation experts ensure to achieve wholistic formulations with better sensory and shelf life.
Working group populations undergo various pressure both from work and family environments. Our formulation experts design food and beverage products or nutraceuticals for stress reduction, including the hypothalamic-pituitary-adrenal axis and sympathetic nervous system. Formation at Guires FRL incorporates a wide range of ingredients that has antidepressant, anti-fatigue and calming properties. This includes vitamin C, milk proteins, a number of herbal extracts (ginkgo biloba, ginseng, kava, valerian and lemon balm), and n-3 fatty acids that have demonstrated potential stress reactivity-lowering and mood-enhancing effects. We are also working on Para probiotics to target weak immunity and the elderly population. This is an “inactivated microbial cells (non-viable) to enhance the immunity, exhibit anti-inflammatory, antiproliferative and antioxidant properties and exert antagonistic effect against pathogens.
Guires FRL formulation experts formulate a wide variety of supplements and sports foods/drinks targeting middle-aged adults involved in Sports. Our product ranges include isotonic drinks as replenishment of water; high carb, citric acids (Vitamins C and E, Carotenoids, Flavonoids, Carnosine, Anserine) for improvement of endurance and prevention of muscle/joint injuries or fatigue; protein, BCAA, Creatine, β-HMB to enhance muscle strength, vitamin C and E glutamine for prevention of a decrease in immunocompetence.
Guires FRL formulation experts formulate products to alleviate depression, anxiety or stress in individuals predisposed to a mood disorder. We formulate products with prebiotics, probiotics, dietary fibre and omega-3-polyunsaturated fatty acids. We also consider Fructooligosaccharides and galactooligosaccharides while formulating the product.
The prevalence of chronic diseases such as diabetes, coronary heart disease, rheumatic heart disease, peripheral arterial disease, diabetes and cancer is high among middle-aged adults. This is due to inflammation-induced endothelial abnormalities and poor dietary habits along with unhealthy lifestyles. Phytochemicals, in general, are evidenced to improve human health as fresh plant products like fruits & vegetables, or at the other extreme as high value-added processed forms. We develop functional foods and beverages and nutraceuticals in the form of dietary supplements, herbals and nutrients. Our team has an experience in formulating products using ketogenic diets, whole grain components, phytoestrogens, peptides, hydrolysates, dairy foods, polyphenols (phenolic acids, flavonoids, stilbenes, lignans and polymeric lignans), prebiotics, probiotics, spices, dietary fibre, postbiotics, PUFA
We develop products using wholesome ingredients that are rich in vitamin D and zinc, omega 3, high fat, vitamin E, whole grains and complex carbohydrates, calcium and protein to meet the nutritional needs of middle-aged adults. We also adhere to the latest technology and processing method while at the same time maintaining shelf life and an appealing appearance, texture and taste. Guires's scientists and formulation experts develop products using natural ingredients or different treatment processing methods and technology based on the requirement. However, we ensure to deliver safe and quality products retaining the best possible natural ingredients during our processing method.
We can formulate the products in the form of ready-to-drink, ready-to-serve, ready-to-eat, ready-to-cook, ready-to-drink, ready-to-mix protein powders, meal replacement drinks, meal kits, low carbohydrate ingredients, sports bars and formulations along with powder form, puffed form, gummies, soft or hard gels, capsules, tablets, syrups, gummies, or sachets containing a concentrated source of minerals, vitamins, or botanical extracts. Our product formulation experts ensure to portray the foods as more enjoyable and riper through hedonic and palatability appeals.
Guires's scientists and formulation experts develop a highly functional strength product that provides the intended results. We synthesise analogues to enhance the efficacy of a promising nutraceutical or identify molecular targets of known nutraceuticals and look for synergistic effects on chemoprevention or chronic disease prevention by using two or more nutraceuticals or derivatives. We adhere to the international guidelines and formulate our product at the same time meet the dairy-free claim as well. Our formulation experts ensure that the end product would meet the required pH, dispersibility, efficacious dosage, shelf life, glycemic index, desired satiety, mouthfeel, flavour, odor and taste. Through years of expertise and knowledge, we bring in the right solutions that fit your requirements.
Any ingredient incorporated into the formulation needs to achieve its intended function in the human body at the same time and must adhere to safety, product purity (i.e., clean label), and developmental appropriateness. At Guires, we substantiate this from many different types of investigation, including secondary research for existing ingredients or, if it's novel, publishing the work in reputable, peer-reviewed journals. Data for this will be derived from chemical comparisons and analysis, activities (e.g., ORAC or oxygen radical absorbance activities), testing in animal models, genomic/gene expression studies, in vitro bioassays and, most compelling of all, human clinical testing. Through years of expertise and knowledge, we bring in the right solutions that fit your requirements.
The viability of microorganisms varies depending on many conditions, such as the characteristics of the microorganism, the acidity degree of the product, the storage temperature, and the characteristics of the packaging materials used. We follow quality measures to ensure the products are safe from both the supplier and the buyer's perspectives. We carry out Marker Chemistry (e.g., HPLC, GC etc.), Microbiology tests (e.g., Pseudomonas, Salmonella, Escherichia Coli), heavy metal analysis (e.g., lead, arsenic, cadmium kept below the regulatory limits), Contamination (e.g., metal flakes from machinery), physical characteristics (e.g., powders for tablets must have low moisture content) and stability (e.g., the time for a product from initial production). We use compendium methods (USP and European Pharmacopeia) for an extensive validation process.
We follow a specific set of regulations that a product must adhere to. This includes manufacturing flow chart, solvents, quantitative ingredient lists, physical characteristics, GMO status, certifications, radiations, pesticide/chemical/ residue, contaminants, microbiology, allergens, animal derivation, and safety (Standard Material Safety Data Sheet – toxicity or clinical testing). We assist ensure to clear the ingredient supplier completes clearance through the target country’s regulatory agency prior to its inclusion in formulas. Through our years of experience, our team can speed up the acceptance and inclusion of new material. Our product development team complies with the Food, Drug and Cosmetics Act (FDA 2009), DSHEA and the Fair Packaging and Labeling Act (Federal Trade Commission 2011), safety and quality food and food supplements guidelines delineated by WHO and the United Nations Food and Agricultural Organization (FAO). Our team ensures to use only acceptable ingredients that are found in the International Nomenclature. We also adhere to the local country’s guidelines as per the client’s request, such as for the Japanese Ministry of Health, Labor and Welfare (MHLW), The Chinese Health Care Association (CHCA), China’s State Food and Drug Administration (SFDA) and FSSAI.
For further information or prices please contact us: