What are Micro Emulsions?
Microemulsions are amphiphile aided thermodynamically stable oil in water (o/w) or water in oil (w/o) dispersions. They have stability for long duration. Normally, an oil-water interface has high interfacial energy so that the free energy of formation of the interface is highly positive. The addition of amphiphilic compounds can bring the interfacial tension to a very low value, leading to spontaneous formation of one dispersion into the other, i.e., forming a microemulsion. The difference between emulsions and microemulsions is in terms of stability. The former has comparatively higher interfacial tension and is kinetically stable, whereas the latter is thermodynamically stable. Thus, emulsions are moderately stable systems and with time separate into water and oil. The droplet sizes of the dispersions in microemulsions range between 10-100 nm; for emulsions the size may be greater than 105 nm. Systems with sizes ranging between 102 to 105 nm are termed as mini emulsions. They also are not thermodynamically stable.
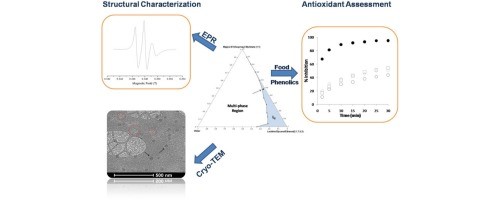
Source: ScienceDirect.com
Certain foods contain natural microemulsions. Microemulsions as a functional state of lipids have been, therefore, used in the preparation of foods. Microemulsions form in the intestine during the digestion and absorption of fat. The possibility of producing microemulsion on purpose and using them as tools in food production is, however, a neglected field in food technology. Excellent component solubilization, enriched reaction efficiency and extraction techniques have considerable potential in the area of food technology. The major differences between food and other microemulsions are in the composition of the oil component and food grade surfactants. In foods, the oil is a triglyceride, whereas in other microemulsions the oil is a hydrocarbon, often a mineral oil. The triglyceride molecule is itself surface active, which in turn implies that triglycerides are not capable of forming separate oil domain in an amphiphile-water system in the same way as mineral oils. Therefore, the composition range in the oil-water-surfactant systems that allows microemulsions to form when the oil is a triglyceride is much smaller than the range allowing microemulsion formation when the oil is a hydrocarbon.
Reference:
https://www.sciencedirect.com/science/article/abs/pii/S0268005X11000397





